[18F]F-FAPI PET/CT to Identify Carcinoma of hitherto Unknown Primary origin: improving minimally-invasive cancer diagnostics.
Status: Open since 12 July 2024. Funded by KWF.
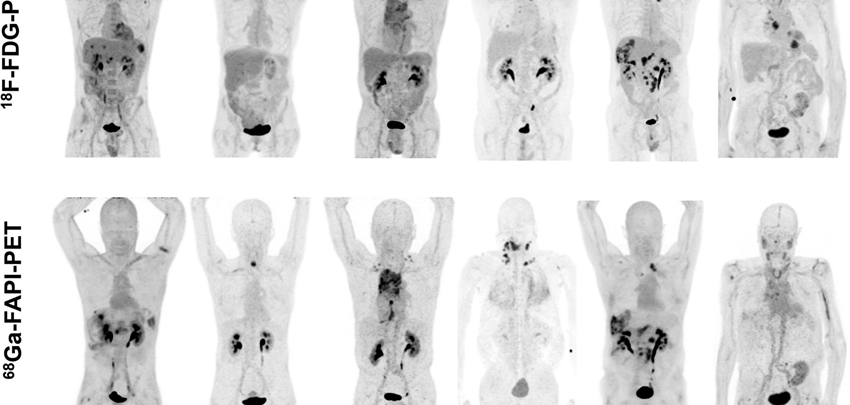
In this non-commercial, investigator-initiated and patient association-driven, multi-center, prospective clinical study we aim to determine the proportion of CUP patients in whom a primary tumor can be identified by F-FAPI PET/CT. We will include 50 patients who have been diagnosed with CUP after the standard diagnostic work-up including FDG PET/CT. Patients will be recruited in six dedicated CUP centers (UMC Groningen, UMC Utrecht, Antoni van Leeuwenhoek hospital, Radboudumc Nijmegen, Maastricht UMC, and Erasmus MC Rotterdam). F-FAPI PET/CT images will be centrally reviewed by 2 independent nuclear medicine physicians/nuclear radiologists. The results from this centralized reading will be made available to the treating physician with an accompanying recommendation for additional diagnostics and/or treatment. Patients will be followed for a maximum of 6 months in order to verify any diagnosis made and to record treatment initiated based on F-FAPI PET/CT. Results of the F-FAPI PET/CT will then be compared to the results from the preceding diagnostic work-up and will be discussed in a multidisciplinary truth committee consisting of at least two oncologists, a pathologist, a radiologist, a nuclear medicine physician, and a methodologist. Identification of a primary tumor is considered a positive outcome of the use of F-FAPI PET/CT. Subgroup analyses will be made based on gender, age, tumor histology, site of metastasis, and performance status (applicable if n>10 per subgroup). The adherence to recommendations based on the F-FAPI PET/CT as well as its additional value in terms of influence on patient management will be evaluated as exploratory endpoints.